Research Interests of the Rohacs lab: Sensory ion channels
Overview:
The goal of my laboratory is to understand the mechanism of the regulation of somatosensory ion channels activated by thermal and mechanical stimuli. We study them at the molecular, cellular and organism level. Our goal is to further the understanding of the fascinating biology of somatosensation, which we believe will ultimately lead to the development of better pain medications with fewer, and less severe side effects.
Somatosensory touch is the least understood of the five senses at the molecular level. Due to the large receptor surface i.e. skin, mucous membranes, bone, etc, direct study of the molecular sensors initiating the detection of mechanical or thermal stimuli has been difficult. Most of our knowledge originates from studying the cell bodies of the primary sensory Dorsal Root Ganglion (DRG) and Trigeminal (TG) neurons. Many of the primary sensors identified by today belong to the Transient Receptor Potential (TRP) family of ion channels; their roles are best established in thermosensation. Piezo channels are the best characterized mammalian mechansensitive ion channels. There are likely to be other, not yet identified primary sensory ion channels. We study both TRP and Piezo channels using electrophysiology, fluorescence imaging, animal behavior, molecular biology and computational modeling.

Transient Receptor Potential (TRP) ion channels
TRP channels have received a lot of attention recently, because of their important roles in a wide variety of important biological processes. Their functions are remarkably diverse; they are involved in temperature sensation, detecting painful stimuli (nociception), vision, taste, Ca2+and Mg2+transport across epithelial cells, apoptosis and Ca2+signaling by hormones and neurotransmitters. Our laboratory focuses on TRP channels involved in temperature sensation and nociception, and on the intestinal and renal Ca2+transporters TRPV6 and TRPV5.
The recent “resolution revolution” in cryo-electron microscopy (cryoEM) produced a large number of beautiful side-chain resolution TRP channel structures. To take advantage of these structures, we collaborate with a cryoEM expert (Vera Moiseenkova Bell) as well as a computational biologist (Vincenzo Carnevale). We aim to develop atomistic models of TRP channel activation and modulation, and experimentally test these models to gain mechanistic insight into how these channels function. These efforts may also lead to developing new pharmacological tools and perhaps better future medications.

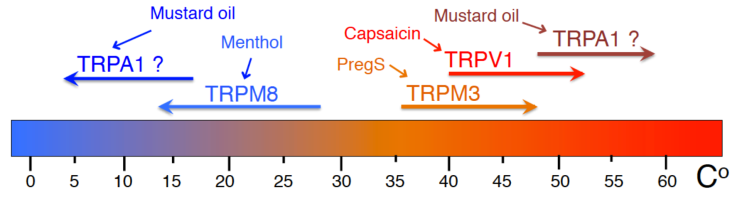
Temperature and pain sensation
Most ion channels involved in detecting environmental temperatures belong to the TRP family, many of them also have naturally occurring chemical agonists, many of which are spices, or chemical irritants. TRPV1 is activated by hot temperatures, capsaicin, the pungent compound in chili peppers, and a number of other pain producing stimuli, such as decreased extracellular pH. Capsaicin, has been used for a long time as a topical analgesic, after an initial burning sensation, it provides pain relief, because TRPV1 becomes desensitized. TRPM8is activated by cold temperatures and menthol. Menthol is also used a topical analgesic, its cooling effect however is transient, again, due to desensitization. TRPM3is another proposed thermosensor, activated by warm temperatures and by pregnenolone sulphate, genetic deletion of this channel leads to impaired noxious heat sensation. TRPA1is a well-established noxious chemical sensor; it was suggested to act as a noxious cold sensor, and more recently as a noxious heat sensor. We study the regulation of sensory TRP channels by cellular signaling pathways using cellular, molecular and in vivo approaches.
Regulation of TRP channels by plasma member signaling pathways
Phosphatidylinositol 4,5-bisphosphate (PIP2) is a minor, but biologically important component of the plasma membrane. This lipid is a common regulator of TRP channels; by studying it, we aim to identify common principles of regulation of this very diverse family of ion channel. We have found that Ca2+influx through TRPM8, TRPV1 and TRPV6 activates a Ca2+sensitive PLC isoform, and the resulting depletion of PIP2leads to desensitization/inactivation of these channels. We are using various cellular and molecular techniques to study the regulation of TRP channels by PIP2, aiming to understand both its physiological relevance and its molecular mechanism.
DRG neurons express a large number of G-protein-coupled receptors (GPCRs). Gs– and Gq-coupled receptors, such as prostaglandin and bradykinin receptors are activated by pro-inflammatory mediators, and they sensitize the pain pathway. Activation of Gi-coupled receptors, such as opioid, somatostatin or GABAB receptors on the other hand generally inhibit the pain pathway. We study how activation of these receptors modulates sensory TRP and other ion channels in DRG neurons. Selective modulation of GPCRs in the peripheral pain pathway can potentially lead to novel pain medications with fewer and less severe side effects.

Mechanosensitive piezo channels
Piezo1 and Piezo2 are bona fidemechanically-gated ion channels, identified in 2010. They are very large proteins, with 38 transmembrane domains and they share no homology to any known protein. Piezo2 is involved in light touch and proprioception as well as in sensing airway stretch. Piezo1 is important for red blood cell function, lymphatic and vascular development, as well as sensing sheer stress in the vasculature. Our laboratory is working on understanding how these mechanosensitive ion channels are regulated by plasma membrane receptors and by phosphoinositides.

TRPV5 and TRPV6
TRPV6 and TRPV5 are Ca2+selective ion channels mainly expressed in the duodenum and distal tubules in the kidney, respectively. They play major roles in organims level Ca2+homeostasis. Both these channels are constitutively active, and their activities depend on the presence of PI(4,5)P2. Our laboratory studies how plamma membrane phosphoinositides and calmodulin regulate these channels, using molecular biology and electrophysiology, in collaboratin with structural, and computational biologists. We are also involved in developing new pharmacological tools to inhibit or stimulate these channels.

